TURALIO is a medication that blocks some of the signals that can cause TGCT.
TURALIO was studied in a clinical trial of 120 patients who had advanced TGCT, for whom surgery was not recommended.
For 24 weeks, half of the patients in the trial were given TURALIO and half were given a placebo. After 24 weeks, patients in both groups were offered continued treatment with TURALIO.
The study tested whether
TURALIO was able to:

Eliminate or reduce tumor size

Increase joint movement
The clinical trial included TGCT in both the upper and lower body
The image below shows the locations of patients’ tumors in the trial (120 patients).
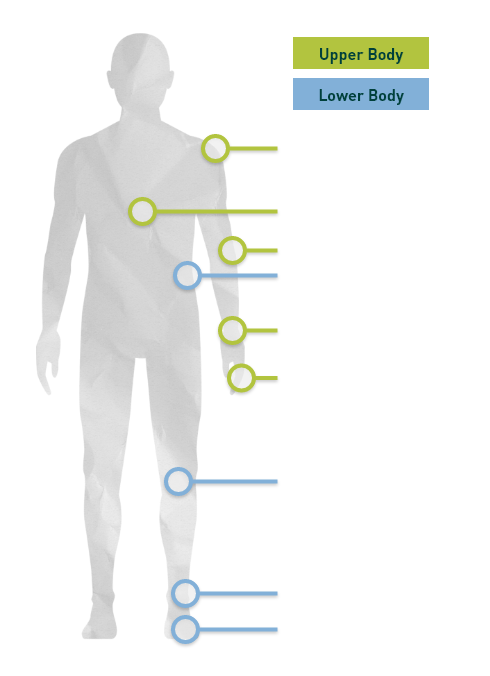
Of patients in the trial:




TURALIO: Clinically proven to help reduce tumor size
Two measurements tracked whether TURALIO was able to reduce the size of the patients’ tumors. One measured the length of the tumor. The other measured the volume of the tumor (the amount of space the tumor filled within the joint).
Results with TURALIO
When measuring by tumor length, 38% people saw their tumors get smaller or disappear completely after 6 months with TURALIO
Of the patients taking TURALIO:
- 23% (14 of 61) had their tumors reduce in length by 30% or more
- 15% (9 of 61) had their tumors disappear completely
No patient taking placebo experienced a reduction in tumor length.
When measuring by tumor length, 61% of people saw their tumors get smaller at completion of the trial at ~5.5 years
Of the patients taking TURALIO:
- 61% (37 of 61) had their tumors reduce in length by 30% or more
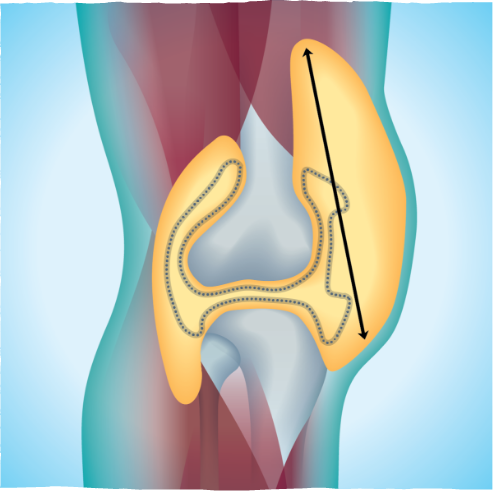
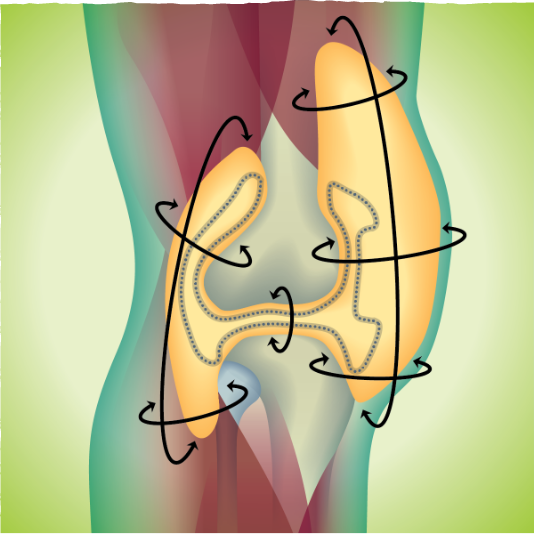
When measuring by tumor volume, 56% of people saw their tumors get smaller by half or more after 6 months with TURALIO
Of the patients taking TURALIO:
- 51% (31 of 61) had their tumors reduce in volume by 50% or more
- 5% (3 of 61) had their tumors disappear completely
No patient taking placebo experienced a reduction in tumor volume.
When measuring by tumor volume, 68% of people saw their tumors get smaller at completion of the trial at ~5.5 years
Of the patients taking TURALIO:
- 68% of patients (62 of 91) had their tumors reduce in volume by 50% or more
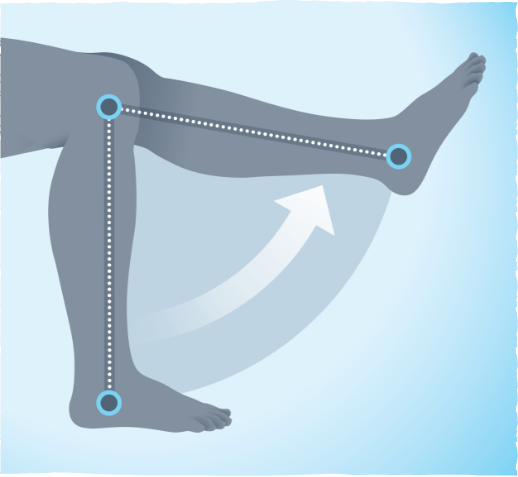
People taking TURALIO had significantly improved range of motion compared to patients taking placebo at 25 weeks
Range of motion is a measure of the amount of movement possible in a specific joint.
Joints studied in the trial included:
- Knees (25 on TURALIO vs 28 on placebo)
- Ankles (11 on TURALIO vs 7 on placebo)
- Other (9 on TURALIO vs 8 on placebo)
Information was not available for 16 of 61 patients in the TURALIO arm and 16 of 59 patients in the placebo arm. It was not possible to know if the patients with data missing were experiencing worse range of motion than patients with data available.
68% of patients (62 of 91) had their tumors reduce in volume by 50% or more.
Preparing to take TURALIO
Discover the first FDA-approved, oral treatment for certain adults who have TGCT that is not likely to improve with surgery.
Learn how to prepare





